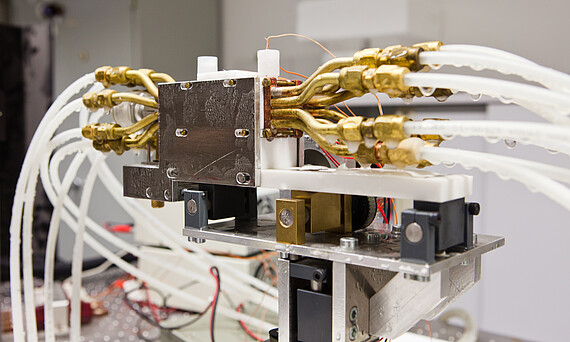
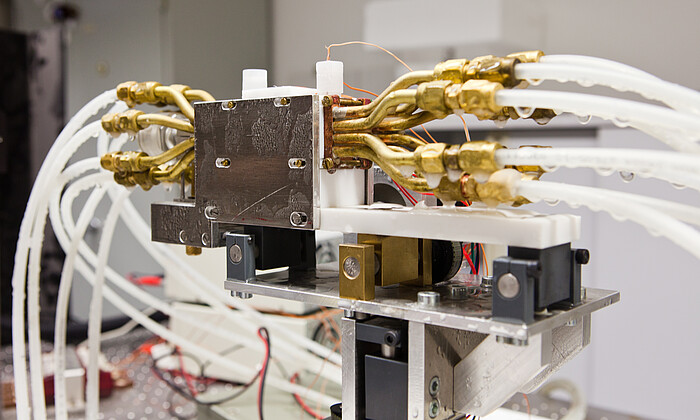
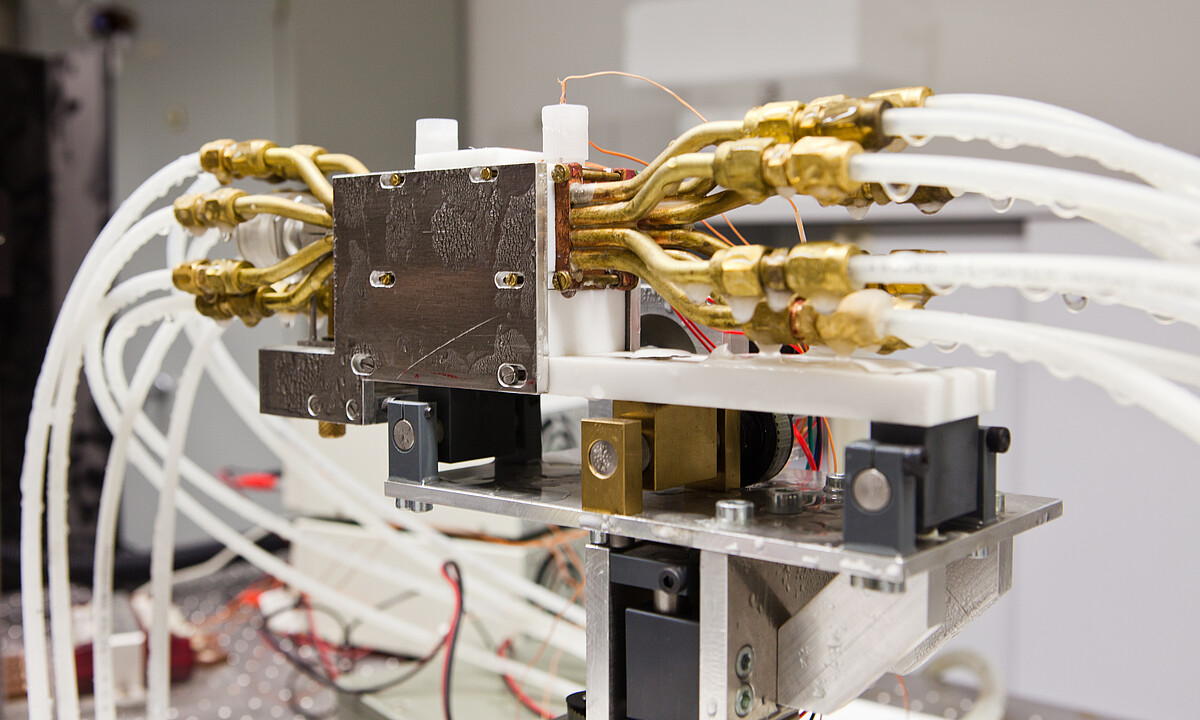
Cryopreservation is a technology that allows for the long-term preservation of various cell types and three-dimensional tissues, both natural and artificially produced, at extremely low temperatures. Alternative cryopreservation strategies are needed due to the cytotoxic effects of widely used cryoprotectants (CPAs) such as dimethyl sulfoxide (DMSO), which depend on time, temperature, and concentration. This can result in decreased vitality and functionality, as well as genetic and epigenetic instability of the cells after thawing. Biorefrigeration technology is also being researched for the storage of artificially produced three-dimensional tissues. Recent developments in tissue engineering for cell and organ transplantation, as well as protective methods such as embedding or encapsulation, offer promising opportunities for the storage and transportation of stem cells and therapeutic products.
The main topics of the Low Temperature Applications/Cryogenics (FTAK) working group are as follows:
-
Methods and devices for efficient freezing and thawing
Since the outcome of cryopreservation is cell-type dependent, the main freezing and thawing parameters must be optimized for each cell type and tissue. The available methods include both direct observation of freezing/thawing processes and observation of the induced ice formation under the cryomicroscope.
- Cryomicroscope with LINKAM
- Cryostage Freezers with controlled cooling rates:
- Commercial (Asymptote VIA Freeze Research, Planer Kryo 560-16, Askion Workbench C-Line WB230, CM2000)
- Developed at the Institute (IMP)
- Defrosting apparatus
- Commercial water baths and automated in-house developments
- Freeze drying
- SP Scientific AdVantage Plus 2.0
- Martin Christ Epsilon 2-10 D freeze dryer
- Directed solidification
- Power down
- Bridgman
-
Induced ice formation
The temperature at which ice forms is a crucial factor that affects the outcome of cryopreservation. To ensure precise control over ice nucleation, we are exploring various techniques, including contamination-free induced ice formation using laser, Peltier electrofreezing
- Contamination-free induced ice formation utilizing laser
- Peltier electrofreezing using a freezer with precisely controlled cooling rates
- Induced ice formation using ultrasound
- Seeding using cold spot ice nucleation (under the cryomicroscope)
-
Safe and low-toxicity cryopreservation
Although cryopreservation is widely used in industrial, clinical, and academic applications, the effects of cryopreservation on subcellular interactions and epigenetic mechanisms have not been clearly characterized. Previous research at the institute has shown that DMSO can affect the genetic and epigenetic profile of cells. These changes may not occur immediately after thawing and could have long-term effects. To mitigate the negative effects of CPA, we prioritize the following:
- New CPAs blends to achieve a reduction in DMSO concentration
- Serum-free cryopreservation
- Cell protection through alginate capsules
-
Physical and biological aspects of cryopreservation
The effectiveness of optimal freezing protocols depends on various physical and biological parameters specific to each cell type. These parameters include cell cycle and age, membrane permeability, interaction of CPAs with the cell membrane and water, and the osmolality of the cryoprotective solutions. In this context, we examine:
-
Heat and mass transport
-
Cell response to CPAs and low temperatures: analysis of membrane permeability (cryomicroscope), osmotic pressure (osmomat)
-
Epigenetic and genetic stability
-
-
Cryopreservation of blood
We are also involved in the following projects:
- Development and establishment of a cryopreservation method for animal erythrocytes to set up blood banks for farm animals and small animals
- Development of an automatic freezing apparatus for human erythrocytes
-
Devices for examining vitality and functionality
- Microplate Reader Multiscan EX Cell viability analyzer
- Beckman Coulter Vi-Cell XR
- Particle counter and cell volume analyzer Beckman Coulter Counter Multisizer 3
- Confocal Laser Scanning Microscope Zeiss LSM 510 Meta
- Fluorescent Microscope Zeiss Axiovert 200M with temperature and humidity controlled incubator
Contact
30823 Garbsen


